
Osteoporosis
July 6, 2023
Designing an Effective Non-Drug Treatment Program
Part 3 – Activating Osteoblasts for Bone Building
As we discussed in the first blog in this series, bone mass is maintained by balanced activity in osteoclasts which remove bone and osteoblasts that build it. Aging is associated with a progressive increase in osteoclast bone removal and diminished osteoblast bone building and repair. Over time, that diminishes bone density. In the last post we discussed the relationship between inflammation and activation of osteoclasts resulting in accelerated bone loss.
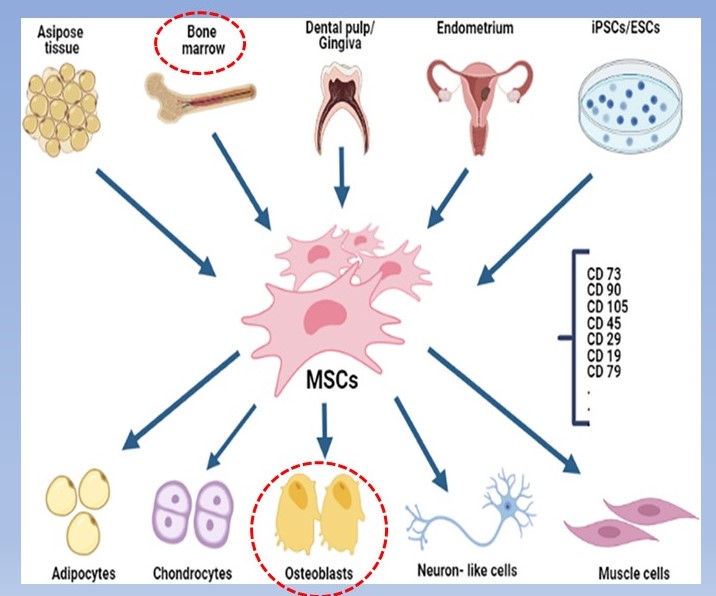
The other piece of the bone loss is diminished bone repair and building by osteoblasts. Two important factors result in this state. The first is declining numbers of functioning osteoblasts. As older osteoblasts die off their numbers must be constantly replenished by mesenchymal stem cell (MSCs) migration to bone. MSCs originate from several sources but the majority come from bone marrow and fat tissue. When attracted to injured or degenerating tissue, they have the unique ability to turn into new cells of that tissue which carry out repair.
Because MSCs can migrate to several types of tissue to initiate repair they need a “homing signal”. This signal in bone is from bone morphogenic proteins (BMPs) which are secreted from the local remaining osteoblasts and the older osteoblasts called osteocytes that are imbedded deep in the bone tissue.
As both of these cell populations diminish with age there is less homing signal for MSCs to migrate to specific bone sites. This is further complicated by aging of bone marrow where fewer stem cells are being generated. Regenerative therapy for large bone defects have used combinations of implants of unmineralized bone fibrous matrix combined with injections of BMPs and MSCs. More recently, supplemental BMPs have become available which is more suited to broad areas of bone as occurs in osteoporosis.
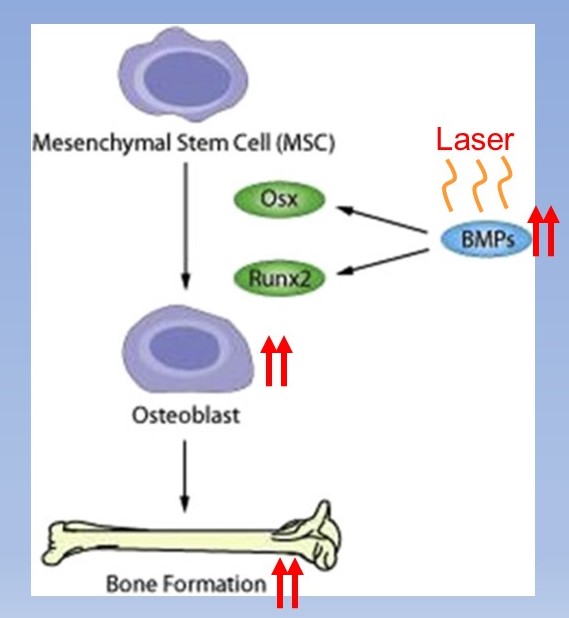
Supplementation of BMPs increases homing of MSCs to the weakened bone. This can be augmented by laser therapy of the weak bone site which increases the release of BMPs by the remaining osteoblasts and osteocytes. Between supplementing with BMPs and increasing local production with laser, MSC attraction can be normalized even with age.
That leaves another issue, the declining numbers of MSCs in bone marrow that are available to migrate to the osteoporotic sites. Laser therapy comes in here again.(1, 2) In addition to applying laser to the weakened bone to increase BMPs, the therapy applied to an area where it is easy to irradiate bone marrow such as along the shin increases MSC release.
There is yet another piece to the task of increasing osteoblast numbers and activity in bone repair, improving the function of some of the aging osteoblasts. A share of the existing osteoblasts in osteoporotic bone function very poorly. This is the result of the accumulation of poorly functioning mitochondria.
As we discussed in a prior blog, cells contain many mitochondria which convert dietary energy such as glucose to cell energy, ATP. As mitochondria age they begin to function poorly producing high amounts of injurious free radicals and little ATP. The poorly functioning ones are normally removed from the cell by autophagy, a process where they are incapsulated and dissolved by enzymes restoring normal cell function. Unfortunately, autophagy declines with age allowing poorly functioning mitochondria to continue producing free radicals which injure the cell further.
Laser therapy, also called photobiomodulation, has also been shown to restore normal mitochondrial ATP production which is likely related to stimulating autophagy.(3) It is a complementary therapy to the autophagy stimulating effect from supplementation of urolithin A we discussed in the last blog.
To summarize to this point, osteoclast bone destruction is elevated in osteoporosis the result of higher levels of inflammation associated with aging. Addressing the sources of higher inflammation can help to slow osteoclast activity. At the same time, diminished bone repair by osteoblasts requires stimulation. Supplementation with bone morphogenic proteins (BMPs) to act as a homing signal to migrating stem cells is very helpful. BMP levels can also be increased with laser therapy to the osteoporotic sites. Laser therapy to an accessible site in bone marrow such as the shin increases stem cell release to migrate to osteoporotic bone to regenerate fully functioning osteoblasts.
We have discussed a lot in this series on osteoporosis and osteopenia. It is always good to then summarize all of this and put it together in a step-by-step program. That we will do in the last blog in this series.
- Bayat M, Jalalifirouzkouhi A. PRESENTING A METHOD TO IMPROVE BONE QUALITY THROUGH STIMULATION OF OSTEOPOROTIC MESENCHYMAL STEM CELLS BY LOW-LEVEL LASER THERAPY. Photomed Laser Surg, 2017;35(11):622-628.
- Yin et al. LOW-LEVEL LASER EFFECT ON PROLIFERATION, MIGRATION, AND ANTIAPOPTOSIS OF MESENCHYMAL STEM CELLS. Stem Cells Dev, 2017;26(10):762-775.
- Hamblin M. MECHANISMS AND MITOCHONDRIAL REDOX SIGNALING IN PHOTOBIOMODULATION. Photochemistry and Photobiology, 2018;94:199–212.