
Osteoporosis
June 22, 2023
Designing an Effective Non-Drug Treatment Program
Part 2 – Inflammation and Bone Loss
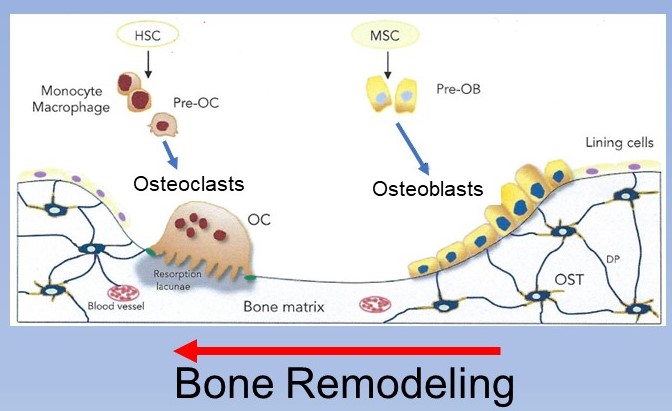
The first post in this series discussed the concept that osteoporosis is a problem of imbalanced cell activity between bone removing cells, osteoclasts, and bone building cells, osteoblasts. Both cells are important for normal bone health and strength giving bone the constant ability to remodel in response to stress by removing bone where not needed and building it up where more stress occurs. This process is a lot like a line from the movie Karate Kid, “Wax on, Wax off” in reverse – “bone off, bone on”. The activity of the osteoclasts removing bone is matched by osteoblasts replacing it with better bone.
Generally, during the first 50 years of life this process is in balance. However, after that point the activities in these cells change with the osteoclasts becoming more active and the osteoblasts becoming less active resulting in net bone loss.
The appropriate issues to address with bone loss are what is driving this altered cell balance and what can be done to change it. The first to address is the bone loss caused by higher osteoclast activity. There are several contributors to this such as declining estrogen levels but that does not tell the whole story. All women will have significant drop in estrogen levels associated with menopause, yet some develop osteoporosis and some do not. Men also produce estrogens from enzymatic conversion of testosterone, but they are less dependent on estrogen function. This difference between women and men is a primary factor in the different rates of osteoporosis which is 4-5 times more common in women.
Declining estrogen may be looked at as more the “final insult” to bone maintenance and not the primary cause of bone loss. The earlier and larger insult is inflammation induced increased activity of osteoclasts and suppression of osteoblasts. This relationship has been understood for quite some time. The high inflammation in autoimmune diseases such as rheumatoid arthritis is well known to induce bone loss.
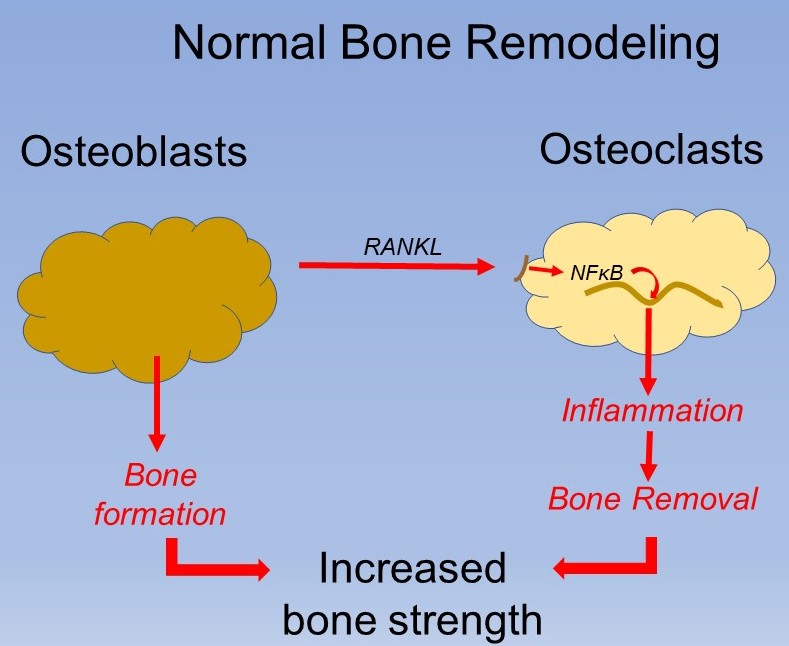
Osteoclast development and activity is stimulated by activation of inflammation. In younger, healthy bone this inflammatory signal actually comes from osteoblasts secreting RANKL which activates NFκB which creates the activation of inflammation. In this case however, the increased activation of bone removal is matched by higher bone building by the osteoblasts. Unfortunately, any chronic activator of inflammation can cause activation of osteoclasts without the appropriate increased activation of osteoblasts resulting in ongoing bone loss. This progressive activation of osteoclasts and bone removal unfortunately occurs while osteoblasts are diminishing in numbers and activity creating a “double penalty”.
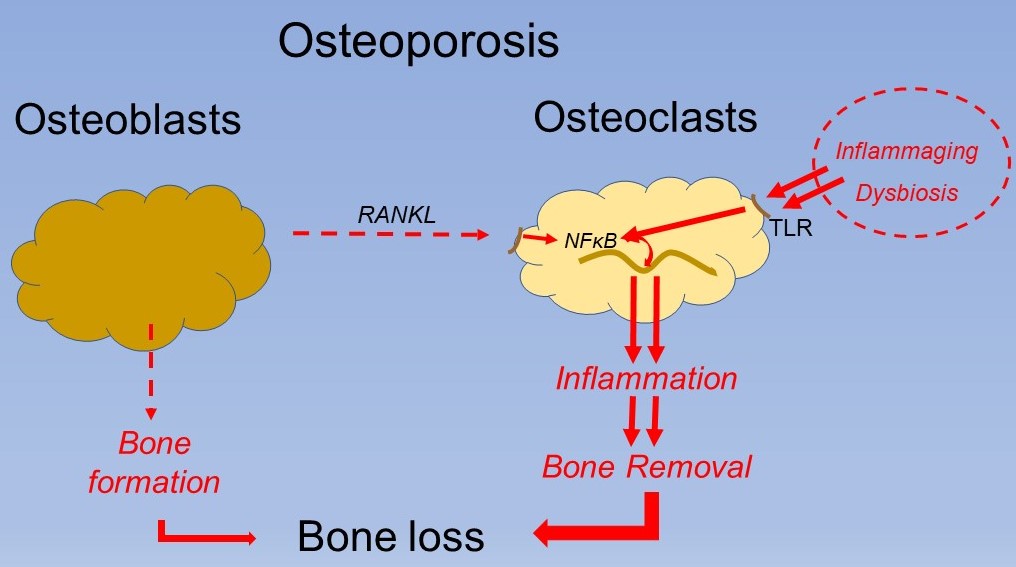
There are many causes of chronic inflammation but perhaps the most related is ageing. Almost all diseases which become progressively more common with age involve inflammation which begs the question, “what increases inflammation with age?”
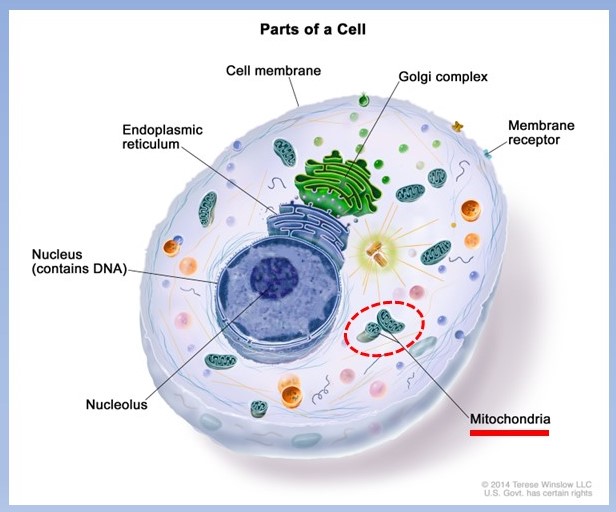
This process has been termed inflammaging which is defined as “A chronic, sterile, low-grade inflammation that develops with age, in the absence of overt infection, and may contribute to clinical manifestations of other age-related pathologies.” The primary driver of inflammaging appears to be the accumulation of old mitochondria which no longer function. Mitochondria are the engines that produce energy in cell. They take diet energy such as glucose and convert it to ATP which is cell energy. Failing mitochondria do not complete the process and release high amounts of free radicals which trigger inflammation.
These dysfunctioning mitochondria are normally removed efficiently by a process called “autophagy” where they are enzymatically broken down and removed from the cell to allow it to function normally. Aging begins to slow autophagy generating more ongoing inflammation. Considerable research in this area is demonstrating that more normal autophagy can be improved by several phytonutrients including quercetin and resveratrol.(1) These nutrients are plentiful in foods such as onions, berries, grapes and others.
Perhaps the best phytonutrients for the restoration of autophagy are ellagitannins which are rich in pomegranate, berries, and walnuts. The ellagitannins are converted to a metabolite urolithin A by the gut bacteria. Higher blood levels of urolithin A have been shown to reduce age related inflammatory markers by improving cell removal of defective mitochondria.(2, 3)
Unfortunately, the age common changes in the microbiome tends to reduce the conversion of ellagitannins to urolithin A. Eating a diet rich in these phytonutrients helps, but once osteoporosis exists, supplementation with higher levels is necessary. Once age related bone loss is occurring, it is best to take a supplement of urolithin A to enhance removal of old mitochondria.
In addition to reducing inflammation in immune cells, autophagy in osteoblasts allows them to function more normally in bone generation. Restoring autophagy has this dual benefit of reducing inflammation induced osteoclast activation and increasing osteoblast activity.
The second source of higher inflammatory activity with aging can come from a chronic immune response to micro-organisms such as bacteria and viruses. With acute infections many of the symptoms experienced are actually not from the infection but rather from the body’s inflammatory response. This inflammation is typically limited to 3-4 weeks until the infection is resolved. Chronic infections, in contrast, produce chronic inflammation. By far the most common chronic infection causing ongoing increased inflammation is an abnormal microbiome in the gut called dysbiosis.
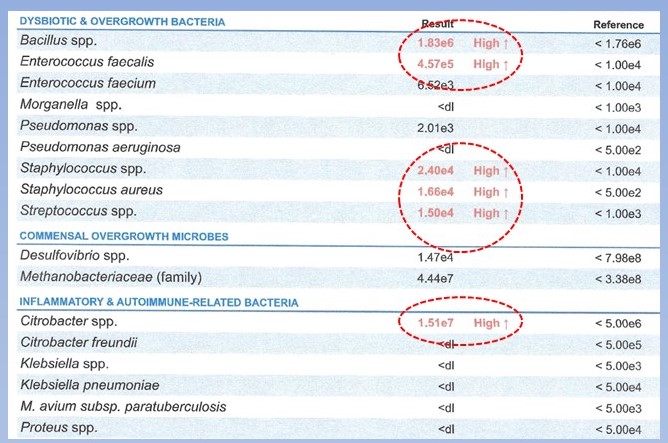
Approximately 100 trillion micro-organisms live in the human body, most in the gut. Some help us decrease inflammation and prevent the overgrowth of potentially inflammatory activating bacteria. Aging is associated with a shift in the microbiome away from the helpful organisms with increased presence of inflammatory inducing organisms, dysbiosis. Symptoms that may indicate dysbiosis include indigestion, abdominal pain/bloating, constipation and diarrhea. About 25% of persons with dysbiosis have no symptoms from the digestive tract but may have other systemic indicators of inflammation like skin disorders such as eczema, joint stiffness, brain fog and others.
Dysbiosis is also clearly associated with osteoporosis, “Although the etiology of osteoporosis and bone loss is multifactorial, recent discoveries highlight the critical role of gut microbiota in bone metabolism. Numerous studies demonstrate the implication of dysbiosis on bone loss and bone disorders through the modulation of the host metabolic system, immune system, and endocrine system.”(2)
Several factors have been associated with this unfavorable shift in our microbiome with age including antibiotic use, antibiotic pass-through in food and poor diet. Generally, we are progressively exposed to more of these factors with progressive age. These microbes are living and need to be fed as we do. They are nourished by fermenting fiber. A byproduct of this fermentation are short chain fatty acids which are anti-inflammatory. This is the synergy that a healthy microbiome creates and is missing in dysbiosis.
Dysbiosis is diagnosed from a stool microbiome analysis. Once the pattern of imbalance is known supplementation with herbal antimicrobials and probiotic combinations can be used to re-establish balance reducing inflammation.
Yes, the balance in bone removal and bone building/repair cell process is somewhat complex, but it is the key to improving osteoporosis. A couple of key questions should arise at this point. The first is why do osteoblasts diminish in function as the osteoclasts increase theirs. The second is what can be done to increase osteoblastic activity and bone generation.
In the next part of the blog we discuss increasing bone repair. Bone building/repair requires the release of bone morphogenic proteins (BPMs) by osteoblasts and precursor cells, osteocytes. BPMs signal stem cells to migrate to the needed area to produce more osteoblasts. We discuss stimulating BMPs and stem cell migration in the next blog.
- Hasima N, Ozpolat B. REGULATION OF AUTOPHAGY BY POLYPHENOLIC COMPOUNDS AS A POTENTIAL THERAPEUTIC STRATEGY FOR CANCER. Cell Death & Disease, 20114;5:1509.
- Singh et al. UROLITHIN A IMPROVES MUSCLE STRENGTH, EXERCISE PERFORMANCE, AND BIOMARKERS OF MITOCHONDRIAL HEALTH IN A RANDOMIZED TRIAL IN MIDDLE-AGED ADULTS. Cell Reports Medicine, 2022;3:100633.
- Lu et al. EFFECT OF UROLITHIN A SUPPLEMENTATION ON MUSCLE ENDURANCE AND MITOCHONDRIAL HEALTH IN OLDER ADULTS A RANDOMIZED CLINICAL TRIAL. JAMA Network Open. 2022;5(1):e2144279.
- Duffuler et al. TARGETING GUT MICROBIOTA IN OSTEOPOROSIS: IMPACT OF THE MICROBIAL BASED FUNCTIONAL FOOD INGREDIENTS. Food Science and Human Wellness2023;13:1-15.