
The Microbiome as a Key in Health and Disease
Day two of the conference I discussed in the last blog concentrated on the microbiome which is this enormous population of micro-organisms such as bacteria that live in and on us. The microbiome has been known to exist for many decades but was poorly understood until heavily researched in the last 20 years. One of the presenters, Patrice Coni, PhD from Belgium, who is one of the leading scientists in this area discussed the research in this area.
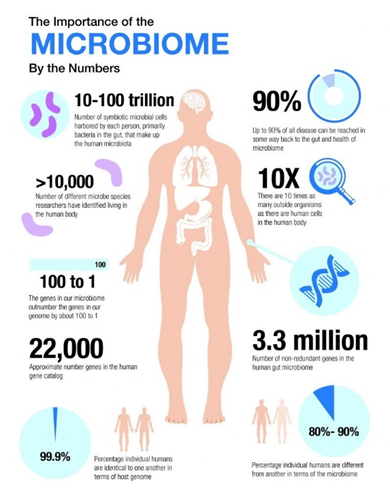
The human microbiome consists of 100 trillion micro-organisms, mostly bacteria, which live in diverse locations on and in us. Most reside in the digestive tract. The size of 100 trillion organisms is appreciated comparing it to our own cell number. For every cell in our bodies there are 10 micro-organisms.
The composition of this microbiome plays an important role in keeping us healthy or promoting disease. Which organism, particularly bacteria, help maintain our health and which contribute to disease is now the focused area of research. That this balance of bacteria is involved both in health and disease is demonstrated comparing the microbiomes of young, healthy mice to that of older, diseased mice.
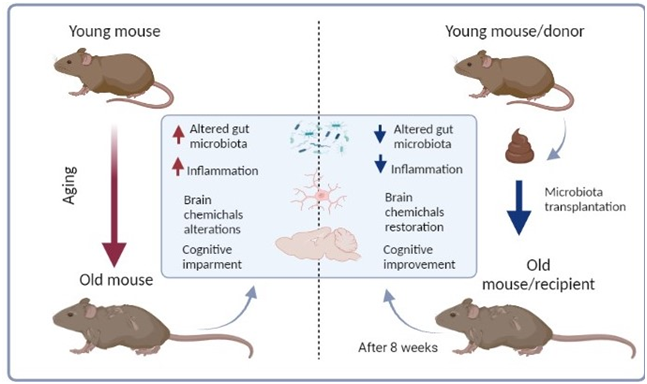
One way to do this comparison is to do fecal medical transplants (FMT) which involve transferring fecal material containing a rich abundance of microbes from young, healthy mice to older, diseased mice. The results are dramatic. The measurable impacts of disease in the older mice such as inflammation, obesity, diabetes, and cognitive impairment improved significantly.
The reverse has also been done giving FMT to young, healthy mice from older, diseased mice. The young mice gradually develop the disease patterns present in the old mice. The conclusion is that, at least in mice, the microbiome plays a large role in the expression of health and disease.
The big question then becomes, what about humans? The answer appears to be the same as with the mice. FMT is approved in the U.S. at this point only for the treatment of unresponsive C. difficile infection which is a difficult to treat intestinal infection. Unresponsive means that previous attempts to resolve it with antibiotics has been unsuccessful. It seems that a good population of the “preferred bacteria” in the intestine can effectively eliminate the C. difficile when antibiotics cannot.
FMT is being used in the research setting to treat a number of aging related diseases. As this evidence grows it is likely to become a very helpful therapy for many if not most. Other researchers are exploring the idea of mining an individual’s feces when they are young and healthy saving it in a biobank for later use should that person become unhealthy.
By now some of you have to be wondering about easier ways to restore a healthy microbiome without FMT. That was the topic of the afternoon sessions which I will discuss in the next blog. As a preview, yes that can be done. Stay tuned.