Peripheral Neuropathy
February 24, 2022
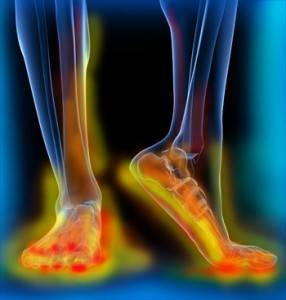
Peripheral neuropathy (PN) is a disorder of nerves within the body, particularly in the legs/feet and arms/hands. Symptoms include:
- Loss of sensation to touch, vibration, and temperature
- Intense pain called neuropathic pain
- Muscle weakness.
The various symptoms relate to the degeneration of different types of nerve fibers within the nerve; motor fibers in the case of weakness, large fibers in the case of loss of touch and vibration, and small fibers in the case of loss of temperature sensation and in neuropathic pain.
Peripheral neuropathy is a huge problem in developed nations. It is estimated that over 10% of the U.S. adult population have the disorder, and this number is an underestimate because many with the disease are only diagnosed later in the process. It is estimated that 60-70% of diabetics will develop peripheral neuropathy at some point, and many will early in the disease. Diabetes is the most common cause of peripheral neuropathy but there are several others.
Up until the past 5-10 years the treatment of PN has primarily involved a combination of managing the cause such as diabetes and pain medications. Managing diabetes, however, does not target improving the symptoms of PN but rather simply preventing worsening. Similarly, pain medications have had several limitations including not reversing the disease process, adverse effects with longer-term use and diminished effect over time.
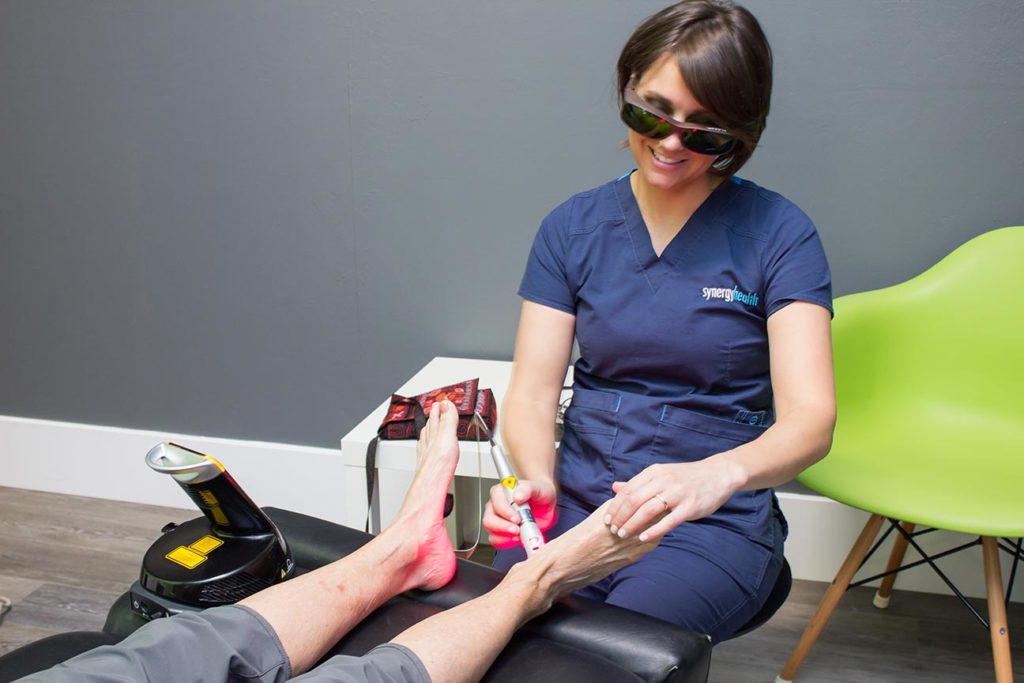
More recently, the treatment emphasis in PN has shifted to therapies which reduce symptoms and result in nerve regeneration and healing. Perhaps the most valuable of these is low level laser also called “cold laser” as it does not produce the intense heat as do surgical lasers.
Many studies have examined both subjective and objective changes in PN after laser therapy. Subjective changes are those such as patient-reported levels of pain. Objective measures are those researchers measured which cannot be controlled by the patient’s response. The primary objective measures looked at are conduction velocity tests which test the speed at which nerve impulses travel. The speed is reduced in PN and that measurement is the standard used to confirm the disorder for diagnosis.
A smaller 2011 study examining 17 subjects with PN confirmed both nerve conduction studies and the Michigan Neuropathy Screening Instrument (MNSI) which is a standardized diagnostic tool combining both patient reported pain scales and examination measurements such as the ability to sense vibration. The treatment protocol used only 10 treatments which has been subsequently shown to be too few to produce maximum benefit. Both the follow-up nerve conduction studies and MNSI score improved.
In a larger study in 2019, active laser therapy was compared to a sham light protocol in subjects with diabetic neuropathy. Three different patient-reported pain and function scales improved between 54 and 183% in the active laser therapy group but not in the sham group. A quality of life scale was also measured and improved 49% in the active laser group. No adverse effects were reported.
Two more studies in 2020 have found similar results with important improvements in reported pain, function and objective testing measurements. One of the most interesting recent studies looked at why these improvements are occurring. The study looked at neurite regeneration following laser applications. Neurites are the projections from the nerve cell that transfer signals to other neurons and peripheral receptors for sensations and functions such as pain, temperature and muscle function. The neurites are the small projections directly from the nerve cell body as shown in the diagram and at the end of the axon which takes the messages larger distances from the nerve cell.
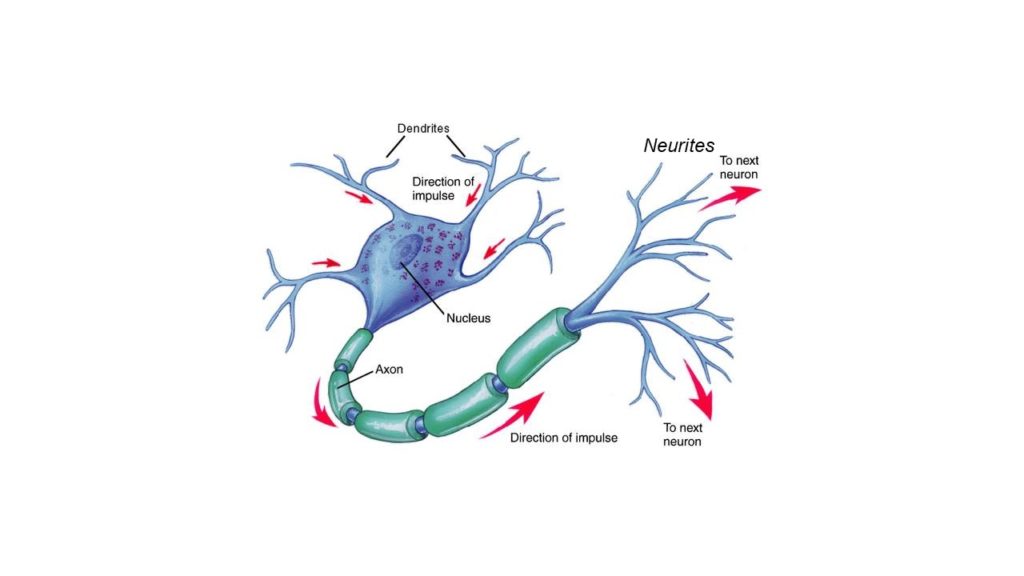
Early in the neuropathy process the neuron begins losing neurites and the protective coating of the long axons, myelin which is shown in the diagram in green. Laser therapy has also been shown to increase myelin repair which is likely another mechanism by which it achieves symptomatic improvement.
Khamshe et al. Diabetic distal symmetric polyneuropathy: Effect of low-intensity laser therapy. Lasers in Medical Science, 2011;26:831.
Chatterjee et al. Effect of deep tissue laser therapy treatment on peripheral neuropathic pain in older adults with type 2 diabetes: a pilot randomized clinical trial. BMC Geriatrics, 2019;19:218.
Shnab et al. The Efficacy of Adding Electromagnetic Therapy or Laser Therapy to Medications in Patients With Diabetic Peripheral Neuropathy. Journal of Lasers in Medical Sciences, 2020;11:14-19.
Rochkind S. Phototherapy in Peripheral Nerve Injury for Muscle Preservation and Nerve Regeneration. Photomedicine and Laser Surgery, 2009;27:219-220.
Diker et al. Comparative effects of photobiomodulation therapy at wavelengths of 660 and 808 nm on regeneration of inferior alveolar nerve in rats following crush injury. Lasers in Medical Science, 2020;35:413–420.