
When Bad Bugs Trigger Food Sensitivities
November 9, 2021
As the awareness of food sensitivities grows, an area of keen interest is about how they happen. For some persons they begin very early in life and likely have a genetic basis. For others they often occur as an adult and gradually foods that had been previously tolerated now cause symptoms. This makes it difficult for some to believe that a reaction to a food is the problem when they tolerated it previously for many years. What may have occurred more recently is a problem called dysbiosis.
Dysbiosis is an imbalance in the microbiome, the organisms living in and on our body. The human microbiome numbers about 100 trillion organisms. To get a perspective on this, for every cell in our bodies which is ours, we have 10 which belong to microbes. This microbiome is spread across many species of bacteria, viruses, and yeast. If they are the right combination, they help us digest and importantly regulate immunity which includes not reacting to self-tissue. With a dysbiotic microbiome the immune system drives inflammation and the many symptoms that can occur when that is excessive. The reaction to many of the dysbiotic organisms in the gut can trigger the release of the protein zonulin.
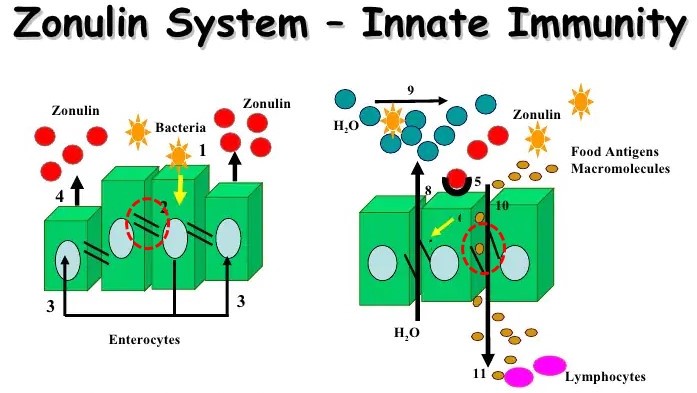
Zonulin was discovered in 2000 by Alessio Fasano, MD in his pioneering work with cholera. It was found to be expressed in reaction to the bacteria cholera causing pain and diarrhea. The cholera toxin activates a receptor in the intestinal surface which triggers zonulin expression inducing the gut barrier to become hyper-porous, aka “leaky gut”. Continuing his research, Dr. Fasano discovered that this same receptor can be triggered by certain food peptides such as gliadin in gluten causing the release of zonulin and the resulting leaky gut. This is thought to be the mechanism by which gluten sensitivity triggers the symptoms.
As more researchers have become aware of this zonulin mechanism, the organisms found to trigger zonulin that induced leaky gut and its symptoms are expanding. Recently researchers found that a peptide in the common microscopic coccidia parasite cyclosporiasis which causes “traveler’s diarrhea” is a potent inducer of zonulin, leaky gut and all of the potential problems that can occur secondary to that. The parasite has a similar surface peptide sequence as gliadin in gluten called a polyglutamate P4022 peptide. Often the onset of food sensitivity follows a period of gastroenteritis where the pathogen triggers leaky gut. This allows the food peptide to get into the second layer of the gut membrane, the lamina propria, which is the immune layer triggering an immune reaction.
One of the growing concerns is the relationship between food sensitivities, leaky gut and autoimmune disease. According to Dr. Fasano who is perhaps the leading scientist in this area, the flood of immune activating substances from both foods and microorganisms into the system from leaky gut causes a “hyper-belligerent” immune system that causes it to turn on self-tissue, the autoimmune process. The most known disease fitting this model is celiac disease. Immune reaction against gliadin in gluten triggers cross-reactivity against an enzyme in the lining of the small intestine, tissue transglutaminase, destroying the intestinal lining. Several studies have found a high association between celiac disease and other autoimmune diseases such as type-1 diabetes and rheumatoid arthritis.
The list of microbes that trigger zonulin release, leaky gut and potentially all of the downstream diseases continues to grow. Dysbiosis is common and is often the triggering factor in the onset of food sensitivities. It can be corrected by a program of “remove, repair and replace” which kills off the dysbiotic organisms, replenishes the helpful gut bacteria and repairs the gut membrane. Most acquired food sensitivities must still be avoided as the immune system remembers and will activate again once it has sensitized to a particular peptide. Restoring a healthy gut and avoiding the acquired food triggers will prevent and often resolve the secondary problems such as systemic inflammation and autoimmunity.
Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol, 2012;10(10):1096-100.
Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020; 9: F1000 Faculty Rev-69.
Lauxmann et al. From celiac disease to coccidia infection and vice-versa: The polyQ peptide CXCR3-interaction axis. Bioessays, 2021, Oct 27;e2100101.
Neuhausen et al. Co-occurrence of celiac disease and other autoimmune diseases in celiacs and their first-degree relatives. J Autoimmun, 2008 Sep;31(2):160-5.